An elusive wreath of carbon has made its long awaited debut.
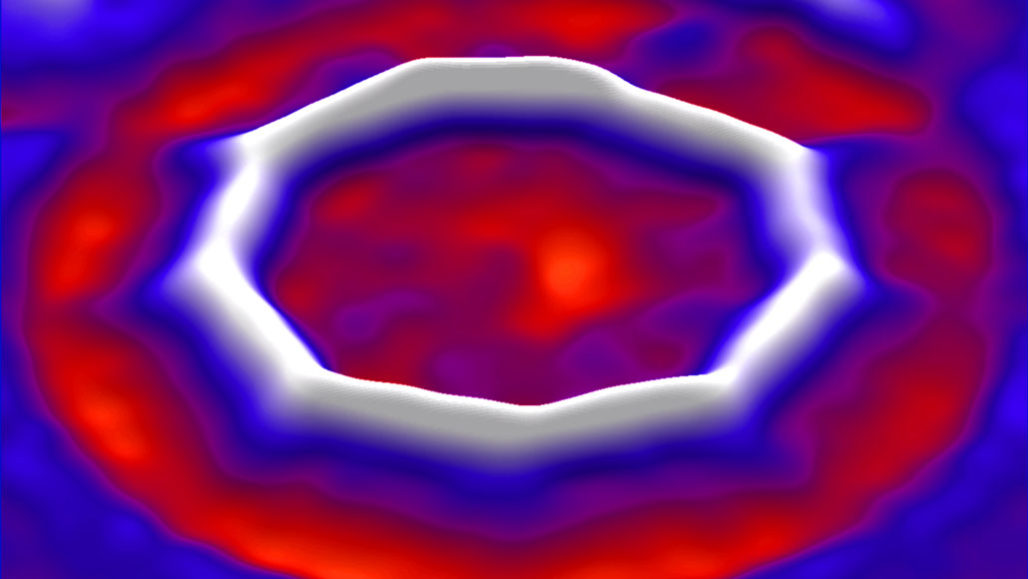
Scientists created a molecule called cyclocarbon and imaged its structure, describing the ring of 18 carbon atoms online August 15 in Science. The work unveils a new face of one of chemisty's most celebrated elements.
Previous research had found hints of cyclocarbon molecules in a gas. But that work didn't satisfy chemists' curiosity because it wasn't possible to image the molecule and confirm its structure. In particular, it was unclear if the bonds between each atom would alternate between longer and shorted lengts, known as single and triple bonds, or whether all the bonds would be the same length, or double bonds. The new study resolves the debate, revealing that the carbon atoms are held together by alternating single and triple bonds.
Previous research had found hints of cyclocarbon molecules in a gas. But that work didn't satisfy chemists' curiosity because it wasn't possible to image the molecule and confirm its structure. In particular, it was unclear if the bonds between each atom would alternate between longer and shorted lengts, known as single and triple bonds, or whether all the bonds would be the same length, or double bonds. The new study resolves the debate, revealing that the carbon atoms are held together by alternating single and triple bonds.
Previous research had found hints of cyclocarbon molecules in a gas. But that work didn't satisfy chemists' curiosity because it wasn't possible to image the molecule and confirm its structure. In particular, it was unclear if the bonds between each atom would alternate between longer and shorted lengts, known as single and triple bonds, or whether all the bonds would be the same length, or double bonds. The new study resolves the debate, revealing that the carbon atoms are held together by alternating single and triple bonds.
Previous research had found hints of cyclocarbon molecules in a gas. But that work didn't satisfy chemists' curiosity because it wasn't possible to image the molecule and confirm its structure. In particular, it was unclear if the bonds between each atom would alternate between longer and shorted lengts, known as single and triple bonds, or whether all the bonds would be the same length, or double bonds. The new study resolves the debate, revealing that the carbon atoms are held together by alternating single and triple bonds.
Previous research had found hints of cyclocarbon molecules in a gas. But that work didn't satisfy chemists' curiosity because it wasn't possible to image the molecule and confirm its structure. In particular, it was unclear if the bonds between each atom would alternate between longer and shorted lengts, known as single and triple bonds, or whether all the bonds would be the same length, or double bonds. The new study resolves the debate, revealing that the carbon atoms are held together by alternating single and triple bonds.




3 Comments
I got this one from 'Related post'
ReplyDeleteThis is a test comment
ReplyDeleteTest is running... >>
Test completed <<
Delete